Trouble shooting tips
Methanol/acetonitrile
Reagent grade solvents contain larger quantity of impurities absorbing UV than HPLC grade solvents do, which makes it difficult to use them in gradient elution or trace analysis. Especially when detection is conducted in a short wavelength, significant differences appear in baseline noise or detection sensitivity. In some cases (or in some wavelengths) it could be feasible to use a reagent grade solvent but we recommend HPLC grade solvents to obtain a stable chromatogram.
Tetrahydrofuran
Tetrahydrofuran easily generates peroxides. To compensate for this tendency, it is commonly mixed with antioxidants. The antioxidants cause a ghost peak so a solvent not containing antioxidants should be used in HPLC. The peroxides in tetrahydrofuran also have great impact on the baseline stability (with differences between grades greater than those of other organic solvents), which prompts a strong recommendation to use HPLC grade solvents with very small quantities of impurities.

Although UV detectors are most commonly used in HPLC, refractive detectors are used when analyzing a compounds without a UV absorption band such as sugars. When conducting sugar analysis using a refractive detector, baseline instability will generally become a problem. Probably most of the baseline drift problems are due to the changes in temperature of a column. Comparison of baseline stability by a difference of a control method of column temperature is shown in the figures below. Baseline drift is very high at an ambient temperature without any column temperature control. Even in the case of a water bath or a column oven, baseline noise may occur by the change of temperature as a result of ON/OFF of a heater. As a measure to avoid this, placing the column in a water bath at the ambient temperature and stirring without heating can produce the desired effect.

Analysis of ionic compounds by reversed-phase HPLC requires the pH control of eluent with acid or buffering agent. The pH control stabilizes the dissociation state of compounds and enhances the reproducibility of retention and separation. Unsuitable pH in analysis causes problems such as a double peak or peak broadening. As shown in the figure below, an identical compound is eluted showing the double peak in pH4.8 eluent, while showing a sharp peak in pH7.0. When analyzing an ionic compound, finding the right pH range for each functional group will result in avoiding such problems.
HPLC analysis of bio samples requires complicated sample preparation depending on the component of interest and sometimes calls for very small amounts of sample for injection. If the injection mass is extremely small in a column in general use with internal diameter of 4.6mm, the component of interest might not be detected as a peak due to diffusion of substance in the column. A semi-micro column with a small internal diameter will be helpful in detecting substances in such small amounts. It is also helpful for eluent saving or application to LC/MS because lower flow rates can be selected in analytical operations.
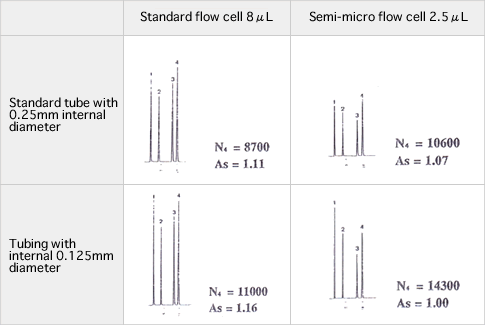
Although the semi-micro column is helpful as mentioned above, care must be taken during operations as described below. Factors causing poor column performance include dead volumes of tubing system including flow cells of the detector.
The figures below depict how internal diameters of tubes and internal volumes of flow cells affect chromatograms. Given the tubing system compatible to analytical columns with standard sizes, column performance (theoretical plate or peak symmetry) degradation occurs due to sample diffusion outside the column. Using semi-micro columns requires suppression of sample diffusion by the tubing system. On the other hand, it should be noted that a small flow cell volume forces a path length to shorten resulting in a lower peak height. It is important to arrange the system environment keeping in mind the considerations discussed above to obtain the column performance and detection sensitivity appropriate to separation of interest.
Are you overly concerned about the degree of absorption when you set the detection wavelength verifying the UV absorption of the component of interest?
Some substances greatly alter their absorption coefficients with a 2 to 3nm variation in the detection wavelength. Moreover, there are instrumental errors. In other words, however precisely you set the wavelength there must be small deviations. To avoid making the 2 to 3 nm deviation matter too much, it is important to choose a stable wavelength with little deviation of the absorption coefficient near the set wavelength rather than choose an unstable wavelength with high absorption.

YMC-Pack Polymine II is highly effective in separation of saccharides including oligosaccharides and is employed in normal phase separation with nonaqueous eluent or in separation of ionic compounds by combining normal phase and weak anion exchange mode.
In the example of analysis of ascorbic acid depicted below, anions in the eluent and amino groups on the surfaces of support material are in a state in which ion-pairs are formed. If the ion-pairing has not yet reached ionic equilibrium, retention times of a sample might alter. We recommend that to avoid this problem by facilitating ion-pairing, the operation as described below be conducted before starting analysis.
Column equilibration in ascorbic acid analysis (in the case the column size 250 X 4.6mm I.D.)
- Flush the columin with water at the flow rate of 1.0 mL/min for 10 minutes.
- Flush the column with 200mM of water solution of ammonium dihydrogen phosphate at the flow rate of 1.0mL/min for 40 minutes.
- Flush the column with water at the flow rate of 1.0mL/min for 30 minutes.
- Flush the column with the eluent for analytical use at the flow rate of 1.0mL/min for 60 minutes.
After using these eluents, sugar analysis using acetonitrile/water eluents might cause anomalies in peak shapes (such as doubling or broadening). Considering the life time or separation reproducibility, it is desirable that separate columns be respectively used for each eluent.
Common problems during HPLC operations include peak shape anomalies such as peak tailing and double peaks. To address these problems, the cause must be precisely determined. The majority of cases are caused by inappropriate conditions of separation which include improper selection of a column or solvent, or deficient columns such as those with void. Here we introduce the method of determining which is the cause.
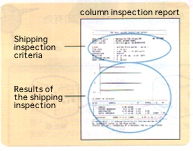
The simplest way is to examine the column performance according to “shipping inspection criteria” in the column inspection report attached to the column. If the examination reveals no peak shape anomalies, the cause will be an inappropriate separation condition. The separation condition such as eluent selection must be reconsidered.
If on the contrary, the same examination reveals any anomaly, the column can be deficient. Flushing (when the impurity could have accumulated) or replacement of the column is necessary. We recommend examining column performances on a regular basis and under the identical conditions.
YMC provide analytical criteria including sample concentrations described in column inspection reports for principal products to help customers examine the performance of the column purchased.
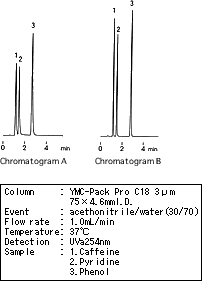
There was a case in which the theoretical plate was reduced and a peak tailing occurred (see chromatogram A) after a conventional column (150 X 4.6mm I.D.) had been replaced with a short column (75 X 4.6mm I.D.) to reduce the analysis time. Although this problem was corrected by replacing the injector, the cause remained unclear. The primary difference between before and after the replacement of the injector was the amount of sample dispersion. The shorter the retention time, the more a peak shape becomes adversely affected by extracolumn dispersion.
As a result, extracolumn dispersion had greater influence on the peak shape with the short column than on that with conventional columns, and caused the peak shape deterioration. Although it is often thought that such problems are due to an insufficient number of theoretical plates and poor peak shapes are caused by a defective column, some cases are caused by hardware problems such as dead volumes of the system. The impact of extracolumn dispersion indeed is often overlooked when using a short column. Even with the short column, a tubing system, an injector and suchlike should lead to minimal extracolumn dispersion.
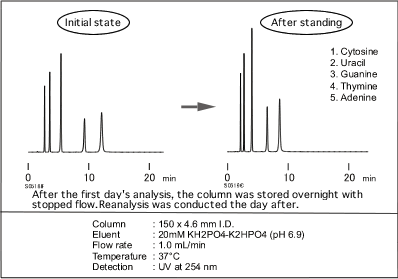
In reversed phase HPLC, column deterioration causes poor peak shapes or shortened retention time. The column deterioration results from packing materials’ chemical alteration such as loss of bonded phase like C18 or dissolution of silica-gel as the base material. Consequently columns in such condition are difficult to restore and reuse.
Meanwhile, 100% aqueous mobile phase in an ODS column sometimes entails steep reduction in retention of compounds as in the figure below. Many may think the reduction of this retention time is due to the column deterioration. However this is not the case. Rather, the cause is considered to be the decrease of apparent hydrophobicity of packing material due to polarity difference between the water of mobile phase and the surfaces of packing material bonded with C18 functional groups and become difficult to solvate. Coping with this and restoring the initial retention time is easily achieved by flushing the column with 10 times its volume of mobile phase containing 50% organic solvent. This situation is believed to result from the decrease of the repulsion between the eluant and the C18 functional groups. If the retention time reduction occurs when using 100% aqueous mobile phase, try to flush with organic solvent/ water mixture to regenerate the column.
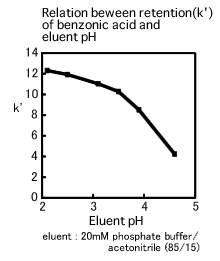
Analysis of ionic compounds by reversed-phase HPLC is conducted with the pH of eluent controlled using acid or buffering agent. However, a separation with a pH range which is not optimum for the compound of interest could cause problems such as double peak or peak broadening. Even if the peak shape is satisfactory, retention time reproducibility could in some cases not be obtained.
The relation between retention of benzonic acid and pH value is shown in the figure below. Although the k’ falls within relatively narrow limits in the region where the pH ranges from 2 to 3.5, it varies widely in the region where the pH ranges from 3.5 to 4.5. The pKa of benzonic acid is 4.2 and it is noticeable that the region where the k’ most widely varies is near the pKa. If the eluent pH is adjusted to the region near the pKa with the wide variation of the k’, the result might not be reproducible since the slight error of the pH adjustment could be of great impact on separation. In fact, the eluent pH variation of just 0.1 significantly affects separation. Consequently, it is desirable that the eluent pH should be more than 1 off the pKa. If the pKa is unknown, the eluent pH should be adjusted to within the region where the impact on separation seems minimal, after having deliberately considered the relation between the eluent pH and the retention time.
When considering the pH value, it is also important to confirm the influence on separation using several eluents with their pHs adjusted to be slightly different from each other.

