AMERICAS
USA
| Show name | Dates | Location |
|---|---|---|
| TIDES | 20 May. – 23 May., 2019 | San Diego, CA |
| PREP 2019 | 7 Jul. -10 Jul., 2019 | Baltimore, MD |
| SFC 2019 | 29 Sep. -1 Oct., 2019 | Philadelphia, PA |
| 2019 EAS | 18 Nov. -20 Nov., 2019 | Princeton, NJ |
| PITTCON 2020 | 1 Mar. – 5 Mar., 2020 | Chicago, IL |
EUROPE
Belgium
| Show name | Dates | Location |
|---|---|---|
| Laborama 2019 | 14 Mar. – 15 Mar., 2019 | Brussels |
Austria
| Show name | Dates | Location |
|---|---|---|
| LAB Supply | 19 Mar., 2019 | Vienna |
Germany
| Show name | Dates | Location |
|---|---|---|
| LAB Supply | 3 Apr., 2019 | Frankfurt-Hoechst |
| LAB Supply | 15 May., 2019 | Leverkusen |
| LAB Supply | 13 Jun., 2019 | Berlin |
| LAB Supply | 28 Aug., 2019 | Dresden |
| BioTalk Summit | 23 Sep. – 24 Sep., 2019 | Berlin |
| LAB Supply | 25 Sep., 2019 | Münster |
| LAB Supply | 29 Oct., 2019 | Hamburg |
| 4. IUTA AnalytikTag | 07 Nov., 2019 | Duisburg |
Italy
| Show name | Dates | Location |
|---|---|---|
| HPLC 2019 | 16 Jun. – 20 Jun., 2019 | Milan |
ASIA
Korea
| Show name | Dates | Location |
|---|---|---|
| The Pharmaceutical Society of Korea | 29 Jan., 2019 | Daegu |
| 2019 KOREA LAB | 16 Apr. – 19 Apr., 2019 | Goyang |
| The Korean Society for Applied Biological Chemistry | 20 Jun. – 22 Jun., 2019 | Busan |
| Korean Chemical Society | 16 Oct. – 18 Oct., 2019 | Changwon |
| The Korean Society of Pharmacognosy | 28 Nov., 2019 | Seoul |
India
| Show name | Dates | Location |
|---|---|---|
| INDIA LAB EXPO | 19 Sep. – 21 Sep., 2019 | Hyderabad |
| CPhI india P-mec | 26 Nov. – 28 Nov., 2019 | Greater Noida |





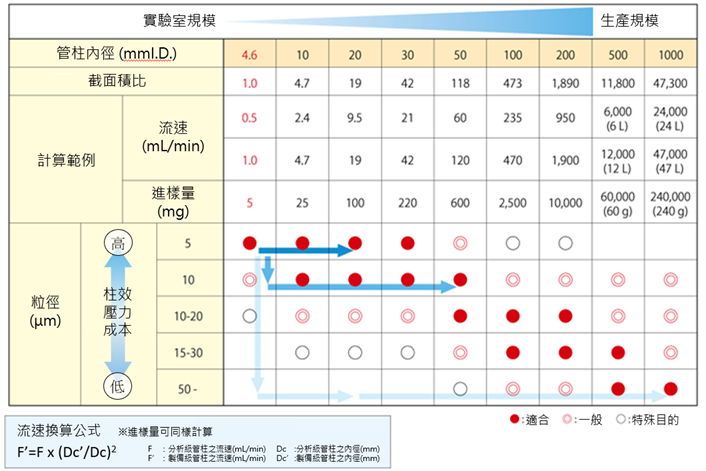
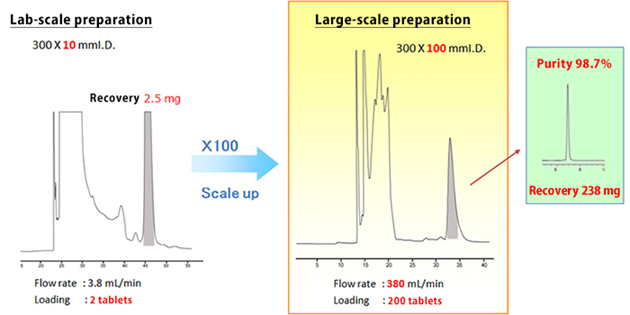

近期留言