選擇溶解樣品之溶劑可能對樣品峰型、分離效率、滯留時間等造成影響。我們選擇Cyproconazole異構物並使用不同樣品溶劑所造成的影響進行說明。詳細說明資料請參考以下連結:


選擇溶解樣品之溶劑可能對樣品峰型、分離效率、滯留時間等造成影響。我們選擇Cyproconazole異構物並使用不同樣品溶劑所造成的影響進行說明。詳細說明資料請參考以下連結:

於1982年,第一個生物相似性藥物—一種透過大腸桿菌(Escherichia coli)複製人類 DNA 而製成的重組人類胰島素上市後,這40年來,全球前 10 種暢銷藥物中有 8 種是生物相似性藥物1。
生物製藥現在占美國食品和藥物管理局(US Food and Drug Administration)每年批准的新藥的三分之一以上。 例如,在 2019 年,FDA 核准五種新的單株抗體(monoclonal antibodies)、三種抗體藥物複合體(antibody-drug conjugates)、三種胜肽(peptides)、一種寡核苷酸(oligonucleotide)、一種小分子干擾 RNA(small interfering RNA)和一種融合蛋白2。
大多數生物製劑仍由細胞基因工程生產。 這些細胞——包括細菌、酵母、哺乳動物和植物——在生物反應器(bioreactor)中生長。收集(harvested)細胞後,Jeffrey A. Karaley*表示:「需要進行純化來移除生物反應器內產生的不想要的物質」。
* Jeffrey A. Karaley, Marketing and Applications Manager for high-performance liquid chromatography (HPLC) and ultra-HPLC(UHPLC) columns at YMC America.
收集的生物製劑在提供給患者之前要經過大約 1,000,000 倍的純化3。純化方法因生物分子而異,但與小分子藥物一樣,層譜在純化的過程中占主要步驟。 生物分子的純化通常比小分子的純化更具挑戰性。原因包括生物分子的體積更大—生物製劑可能比小分子藥物大1000倍—以及結構的複雜性更大。例如,粗(bulk)單株抗體純化通常從親和層析(affinity chromatography)開始,然後是離子交換 (IEX) 層析。 後續的疏水作用層析 (hydrophobic interaction chromatography , HIC) 步驟也經常使用。
HPLC 和 UHPLC 也廣泛使用於整個生物製劑生產過程中的品質控制(quality-control)。 生物分子經常使用體積排除層析(size- exclusion chromatography, SEC)、離子交換層析(ion-exchange chromatography, IEX )、疏水作用層析(hydrophobic interaction chromatography , HIC) 和逆相層析 (reversed-phase chromatography, RP)之分離模式。同樣的技術也用於確保生物製劑在儲存過程中不會降解。
親和層析 AFFINITY CHROMATOGRAPHY
親和層析使用層析樹脂(Resin)和生物分子之間的特異性具可逆性的結合作用來達成分離。相互作用通常包括抗體(antibody)-抗原(antigen)、酶(enzyme)-受質(substrate)和酶(enzyme)-抑製劑(inhibitor)。
對於抗體純化,經常使用Protein A的樹脂。在金黃色葡萄球菌(Staphylococcus aureus)的細胞壁中發現了Protein A,其可與多種單株抗體強力且可逆地結合。因此當粗樣品(crude sample)通過層析管柱時,Protein A會與目標抗體結合,接著可將其他雜質從樹脂上洗出。將緩衝液(buffer)的 pH值降低,可使抗體解除結合並從管柱中洗脫(elution)出來。
Gerard Gach*表示:「自 1950 年代以來,親和層析一直用於抗體純化。自那時以來,市場上的產品一直在不斷發展以滿足客戶的需求。最近,層析設備商需持續跟上製藥產業腳步為生物製劑開發連續式生產製程。」
* Gerard Gach, Chief Marketing Officer at YMC Process Technologies.
連續式生產的目標是將原材料經由單一多步驟流程中生產生物製劑。相較之下,傳統生物性批次生產包含一系列階段步驟,通常在不同階段間有時間間格或地點變化。
成本估算可能各不相同,但普遍認為,連續式生產至少會使生產生物製劑的成本減半4。Gach表示:「FDA 鼓勵生物製劑製造商使用連續式生產來降低成本、增加藥物的可用性並提高產品品質的一致性。」
然而,這種製程方式的重大改變帶來了許多技術和物流的挑戰。儘管如此,生物製程上游製程(upstream stages)包括細胞生長(cell growth)和收集(harvest)正逐漸採用連續式程序5。下游製程(如純化)的進展較慢。Gach 表示:「親和層析是連續程序中一個特別具有挑戰性的步驟。」適合的系統即將上線,例如雙親和層析管柱技術YMC Contichrom Twin CaptureSMB。該系統適用於從實驗室(bench)到生產規模(production scale)的抗體純化(antibody purification)。
YMC Contichrom Twin CaptureSMB 為雙親和管柱系統,適用於從實驗室(bench)到生產規模(production scale)的抗體純化。
Image credit: YMCCo, Ltd.
生物製劑製造商的另一個常見問題是親和管柱與目標生物分子(target biomolecules)的結合效率僅為 40-60%。當推動層析管柱以達到更高的負載時,目標分子往往會直接穿過層析管柱而未被捕獲(uncaptured)。為了解決這個問題,YMC Contichrom Twin CaptureSMB系統串聯兩支親和管柱,這意味著第二支管柱可以捕獲任何通過第一支管柱的目標分子,有效地使第一支管柱的效率達到 95% 或更高。
Gach表示:「YMC系統允許當第二支層析管柱仍在進樣時,第一支層析管柱獨立出流路進行樣品洗脫(eluted)。當第一支層析管柱洗脫完成時,會自動接回流路並獨立出第二支層析管柱進行樣品洗脫。並一直持續這樣的程序。」 這種雙管柱系統已被證明可將產率提高多達三倍,同時相較於傳統批次式製程,只使用約一半的緩衝液和Protein A樹脂。
採用 YMC 技術的製藥公司包括 Bristol-Myers Squibb,該公司已將 YMC Contichrom Twin CaptureSMB 引入其某些抗體療法的臨床生產中6。一家總部位於歐盟的生物製藥公司也購買了雙管柱系統用於優良製造規範生產(good manufacturing practice production), 以及多間歐洲和美國的客戶於試生產(pilot scale)使用7。
YMC 還有其姊妹技術 Contichrom Twin MCSGP HPLC,旨在連續式純化其他生物分子,例如胜肽(peptide)、抗體-藥物複合體(antibody-drug conjugates)和寡核苷酸(oligonucleotides)。
分子篩層析 SIZE-EXCLUSION CHROMATOGRAPHY
SEC按體積尺寸大小對生物分子進行分離,是一種常用來檢測和觀察聚集體(aggregate)的形態的品質控制方法。 在生產和儲存過程中,生物分子有結塊的趨勢,形成二聚體(dimer)和三聚體(trimer)甚至更大的聚集體。Cinzia Stella*表示:「聚集體會導致免疫原性(immunogenicity),我們使用分子篩層析來監控尺寸變異的程度,以確保批次的一致性和產品品質。」
* Cinzia Stella , Senior Scientist in the protein analytical chemistry department at Genentech
用於 SEC 的樹脂(Resin)是高度多孔性(porous)的。 生物分子的大小決定了它是否可以穿透至孔隙中以及多深。由此決定了它從樹脂上洗脫(eluted)的速度。Takashi Sato*表示:「較大的分子無法進入孔隙,只能直接通過層析管柱,而較小的分子則深入孔隙並較晚被洗脫出來。”YMC in Japan 銷售和營銷經理 說。
*Takashi Sato, Sales and Marketing Manager at YMC in Japan
YMC有兩種適用於生物分子的 SEC產品:YMC-Pack Diol 和 YMC-SEC MAB。 第一種有四種孔徑。 60 Å適用於分離較小之生物分子。120、200 和 300 Å 層析管柱適用於分離蛋白質。第二種有 250 Å孔徑,專門設計用於檢測單克隆抗體和抗體-藥物複合體的聚集體和片段。
製造平台是一套完善的流程,適用於一系列產品。這些平台技術在生物製藥製造的大多數階段越來越受歡迎,包括 SEC。例如,適用所有的單克隆抗體使用相同層析管柱和流動相的例行性 SEC 分離方法。Stella 表示:「我們長期以來一直在研究 [免疫球蛋白 G 抗體(immunoglobulin G antibodies)],我們嘗試盡可能多地使用適合我們大多數分子的平台方法。如果可以將相同的方法用於盡可能多的免疫球蛋白 G 抗體產品,而不必每次都開發和驗證新的方法,那麼品質管理實驗室的工作就會變得容易得多。」
然而,平台技術不是靜態的。Stella 表示:「我們緊跟最新的分析技術,如果我們發現有另一種層析管柱比我們目前使用的層析管柱更好,那麼我們會盡可能嘗試更改。」
離子交換層析 ION-EXCHANGE CHROMATOGRAPHY
Kakaley 表示:「離子交換層析對於大多數生物製劑的分析分離和製備純化都很有用。」 這種層析模式經常用於單株抗體的批次式第二步純化,用於親和層析之後。
IEX 層析是根據生物分子整體表面電荷(surface charges)的差異來分離生物分子。該樹脂含有帶電基團(charged groups),可與生物分子表面的帶電氨基酸側鏈形成可逆相互作用。接著利用鹽濃度或 pH 梯度將這些物質從管柱中洗脫出來。
鹽離子與生物分子競爭樹脂上的帶電基團。這意味著隨著流動相中鹽濃度的增加,生物分子將開始脫離樹脂並從層析管柱中洗脫。 具有最低淨電荷(lowest net charge)的蛋白質將首先被洗脫,具有最高淨電荷(highest net charge)的蛋白質最後被洗脫。
對於 pH 梯度,洗脫順序取決於被分離的生物分子的等電點(isoelectric point)。等電點是生物分子上的帶電基團相互抵消時的 pH 值,使該生物分子的總電荷為零。例如,對於陽離子樹脂,當流動相的 pH 值低於生物分子的等電點時,它的總電荷將為正電荷並與樹脂結合。 當流動相的 pH 值升至等電點以上時,生物分子的整體表面電荷將為負; 它將被樹脂排斥並從層析管柱中洗脫。
YMC生產專為蛋白質、胜肽和核酸的 IEX 層析法的親水聚合物珠:YMC BioPro IEX。它可用作陽離子樹脂和陰離子樹脂。這兩種類型各有多孔和無孔的形式產品。多孔樹脂提供了更好的分離的可能性,但同時也要考慮較慢的流速。Sato表示:「非多孔性樹脂特別適合快速分析之應用。」
所有 YMC BioPro IEX 樹脂均有提供分析級層析管柱(analytical chromatography columns)和製備級純化分離(preparative-scale separations)的材料(bulk materials)。
YMCBioPro IEX 樹脂是專為蛋白質、胜肽和核酸的 IEX 層析而定制的親水性聚合物珠。
Image credit: YMCCo, Ltd.
疏水性作用層析HYDROPHOBIC INTERACTION CHROMATOGRAPHY
HIC 利用表面疏水性的差異來分離生物分子。這種模式經常使用在生物分子粗產物純化生產過程的第三步,在親和層析(affinity)和離子交換層析(IEX)之後。
HIC 樹脂含有疏水基團,可與生物分子表面的胺基酸疏水側鏈形成可逆作用。在分離過程中使用鹽類梯度。高鹽濃度會加強生物分子和層析管柱之間的作用力,而較低的鹽濃度則會削弱此作用力。整個生物分子的疏水性越強,促進其結合所需的鹽類就越少。在分離過程中鹽濃度會從高到低,這表示疏水性最低的生物分子會先洗脫(eluted),疏水性最強的生物分子最後洗脫。
這種層析模式在抗體-藥物複合體的品管分析越來越受歡迎。抗體-藥物複合體是單株抗體上藉由化學鍵結的小分子藥物所組成。所需的藥物與抗體比率因治療而異,但平均三或四個分子與單個抗體鍵結的藥物是一個常見的比率8。如果平均比率太低,療效可能會降低,但過高的比例可能會導致服用過量。因此,在所有抗體-藥物複合體的生產過程中,該比率的分析是關鍵的品質控制步驟。
Stella 表示:「我們通常使用 HIC 來分析抗體-藥物複合體的藥物與抗體比率。」基因泰克生產的Kadcyla是一種 HER2-陽性轉移乳腺癌的治療藥物,該藥物於2013年2月獲得 FDA 批准,成為首批上市的抗體-藥物複合體之一。
HIC 適用於抗體-藥物複合體分析,是因為大多數小分子藥物是疏水性的。未結合藥物的抗體會先從層析管柱上洗脫,而連接了最多藥物分子的抗體最後洗脫。比率可以藉由峰面積(peak areas)來確認。
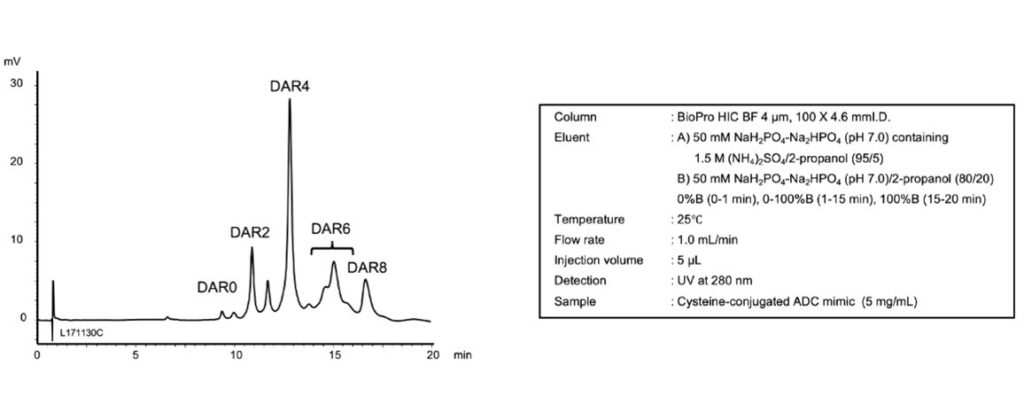
與其他競爭者之層析管柱相比,BioPro HIC BF層析管柱可得到傑出的解析度(resolution),並且對於判斷抗體-藥物複合體的藥物與抗體比率非常好用。
Image credit: YMCCo, Ltd.
2018年,YMC 推出了 YMC BioPro HIC BF材質,針對抗體-藥物複合體、抗體和其他蛋白質分析以及實驗級純化進行最佳化。Sato表示:「我們目前正積極開發另一種新的 HIC分離管柱以實現更快速的分離。」
逆相層析REVERSED-PHASE CHROMATOGRAPHY
RP層析的基本原理與HIC相同。固定相膠體上的疏水官能基團與生物分子表面的疏水官能基團(可逆性)結合。利用有機溶劑的濃度梯度按照生物分子的疏水性強度從管柱上洗脫出生物分子。
然而,用於逆相層析的膠體通常比 HIC樹脂疏水性更強。這導致生物分子和樹脂之間的相互作用更強,需要非極性有機溶劑(如:乙腈)來破壞鍵結並洗脫生物分子。
歷史上,逆相層析不常用於分離大生物分子。 洗脫這些物質需要傳統(純矽膠)逆相層析柱無法承受的條件。導入具有更強化學耐受性的混層樹脂材料(Hybrid resin materials)使這些分離成為可能。例如,近期發表的 YMC-Triart Bio C4 是一種300 Å 多孔性混層樹脂,專為利用RP分離完整單株抗體和其他大蛋白質而設計。
使用混層樹脂的RP層析法也是分離小生物分子的常用工具,YMC-Triart Bio C18專為此目的而設計。YMC-Triart Bio C4和YMC-Triart Bio C18 系列採用 1.9、3 和 5 µm 粒徑生產,可用作分析和半製備級層析管柱。這些層析管柱也非常適合用於胜肽圖譜(peptide mapping)鑑定,這是一種很重要的蛋白質結構確認方法。
結論CONCLUSION
生物製劑將繼續發展。 單株抗體復邁(Humira, 一種由艾伯維(AbbVie)生產的自身免疫性疾病治療藥物)多年來一直是全球最暢銷的藥物。它在 2018 年的全球銷售額為 199 億美元,預計將在 2023 年美國專利到期之前保持其頭把交椅9。另一種單株抗體—默克公司(Merck & Co.)的癌症免疫療法 Keytruda的銷售額預計將超過它。
近年來獲得 FDA 批准的生物製劑類型已從單株抗體擴展到包括更複雜的設計,例如抗體-藥物複合體。與此同時,生物分子製造正在從批量生產轉向連續式生產。層析製造商正在跟上產業變化的步伐,不斷發展樹脂(Resin)並發表適用於有效生物治療浪潮的新型層析設備。
REFERENCES
1. Lisa Urquhart, “Top Drugs and Companies by Sales in 2018,” Nat. Rev. Drug Discovery, 18 (2019): 245, https://doi.org/ 10.1038/d41573-019-00049-0.
2. Lisa M. Jarvis, “The New Drugs of 2019,” Chemical & Engineering News, Jan. 17, 2020,https://cen.acs.org/pharmaceuticals/drug–development/new– drugs-2019/ 98/ i3.
3. “Building Biologics,” Genentech, Feb. 8, 2016, https:// www.gene.com/stories/building–biologics.
4. Randi Hernandez, “Supplement: Integrated Continuous Manufacturing of Biologics,” Genetic Engineering and Biotechnology News, Sept. 14, 2017, https:// www.genengnews.com/magazine/ 300/supplement-integrated-continuous–manufacturing-of-biologics/ .
5. Ned Pagliarulo, “Pharma’s Slow Embrace of Continuous Manufacturing,” BioPharma Dive, Sept. 24, 2018, https:// www.biopharmadive.com/news/pharmas-slow-embrace-of-continuous-manufacturing/ 532811/ .
6. James Angelo et al., “Scale-Up of Twin-Column Periodic Countercurrent Chromatography for MAb Purification,” BioProcess International, April 20, 2018, https:// bioprocessintl.com/ 2018/april-2018/scale-up-of-twin-column-periodic-countercurrent-chromatography-for-mab-purification/ .
7. Kathleen Mihlbachler, Lindsay Arnold, and Jared Steffy, “Continuous Capture Chromatography as Part of an Integrated Downstream Purification Platform for mAbs” (poster presentation, PREP Symposium, Baltimore, July 7, 2019), https:// www.ymcpt.com/sites/default/ files/ PREP%20Poster%20AZ%20YMC%20Twin.pdf.
8. Xiuxia Sun et al., “Effects of Drug-Antibody Ratio on Pharmacokinetics, Biodistribution, Efficacy, and Tolerability of Antibody-Maytansinoid Conjugates,” Bioconjugate Chem. 28, no. 5 (2017): 1371–81, https://doi. org/10.1021/acs.bioconjchem.7b00062.
9. Sarah Houlton, “Back to Basics,” Chemistry World, Dec. 12, 2019, https://www.chemistryworld.com/news/pharmaceuticals–roundup–2019/ 4010812. article.


生產級(Pilot or production scale)/製備型液相層析(Preparative HPLC /MPLC)與實驗室液相層析(HPLC)的分離純化(purification)原理和方法相同,但由於進樣量與層析管柱尺寸大小差異,製備級純化需考慮生產成本並有效收集目標成分(並達到要求規格,例如純度),因此分離純化策略上需與實驗室採取不同的角度來選擇分離條件。
■ 選擇層析分離模式(膠體填料) Separation mode (packing material)
■ 分析條件確認 Separation method
■ 進樣濃度與體積 Concentration Load and Volume Load
■ 進樣量最佳化 Loading amount
■ 檢測條件最佳化 Detection
■ 純化放大規模選擇 Selection guide for the preparative scale
■ 管柱純化膠體之粒徑選擇 Selection of the particle size
■ 分離條件最佳化 Separation method optimization
■ 選擇合適之分離純化收集系統 Preparative system evaluation
製備純化放大之方法說明歡迎來信索取

Nucleic acid therapeutics such as antisense, siRNA and aptamers are expected to play an important role as next-generation pharmaceuticals together with antibody drugs. These drugs demand chromatographic purification and analysis that can recognize slight structural differences following synthesis.
On this page, we provide useful tips for optimization of ion-exchange chromatography methods for oligonucleotides.
Read more “Optimization of oligonucleotide separations on ion-exchange chromatography”


The multifunctional preparative HPLC system, LC-Forte/R is equipped with a recycling function as a standard feature. This enables high purity purification of the compounds which are difficult to separate in a single step even without changing columns and mobile phase. In addition, CHIRAL ART, columns/packing materials with polysaccharide derivatives as their chiral selectors, have a range of packed columns with inner diameters up to 30 mm and are applicable to separations from analytical to preparative scale.
Presented below is an example of high purity preparative purification of flurbiprofen using LC-Forte/R and CHIRAL ART column.

Antisense DNA and siRNA have been widely used for gene silencing in basic research and in medicinal applications.
Effective delivery of the oligonucleotides into cells is important for clinical applications. Previous methods took several hours or more to deliver oligonucleotides to cytoplasm. However, oligonucleotides modified with low molecular weight disulfide units at their terminuses reached the cytoplasm 10 minutes after administration to cell culture 1).
In RP-HPLC using C18 columns, such disulfide modified oligonucleotides show poor peak shape due to too strong interaction between highly hydrophobic their disulfide units and C18 phase. On the other hand, YMC-Triart Bio C4 show a good peak shape because of its short alkyl chains and low hydrophobic interaction.
Oligonucleotides are negatively charged polymers, resulting in low cell membrane permeable efficiency. Various strategies are being pursued including chemical modification of oligonucleotides itself. Recently, it is reported that the disulfide-modified oligonucleotides are efficiently internalized into cytoplasm through disulfide exchange reactions with thiol groups on cell surface 1).
The chromatograms show the separation of phosphorothioate oligonucleotides modified with highly hydrophobic disulfide units. The target disulfide-modified oligonucleotides are not completely eluted from the C18 column due to the strong hydrophobic interaction. On the other hand, a good peak shape is obtained using YMC-Triart Bio C4, which has short alkyl chains, by the moderate interaction.
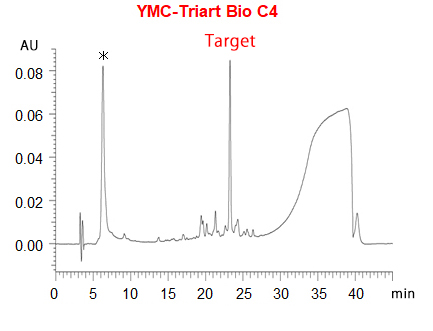
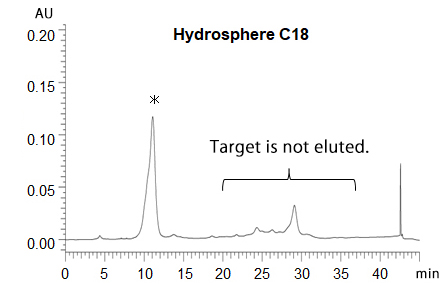
*phosphorothioate oligonucleotides without disulfide-unit

| Column | 5 µm, 250 X 4.6 mmI.D. A) 50 mM TEAA* (pH 7.0)/acetonitrile (95/5) B) acetonitrile 5-95%B (0-30 min), 95%B (30-35 min), 95-5%B (35-35.1 min), 5%B (35.1-45 min) |
|---|---|
| Flow rate | 1 mL/min |
| Temperature | 50°C |
| Detection | UV at 260 nm |
| Sample | crude reaction mixture |
* triethylammonium acetate
Courtesy of Saki Kawaguchi, Chemistry Department, Nagoya University, Japan

During manufacture and/or storage of biopharmaceuticals, variants with different properties from desired substances are produced by enzyme reactions or physicochemical interactions. Characterization of the variants is of great importance from the perspective of ensuring efficacy and safety of pharmaceutical products.
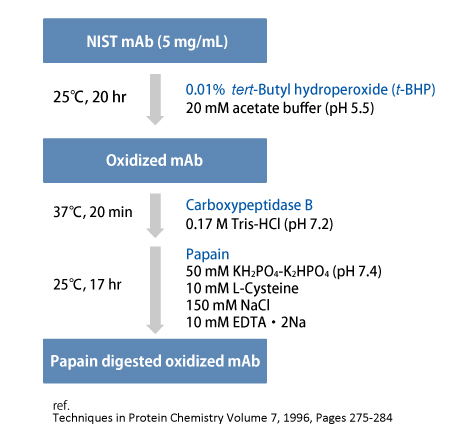
Oxidized mAb variants can be analyzed by hydrophobic interaction chromatography (HIC). On this page, we introduce the separation of mAb samples and their oxidized species using our HIC column, BioPro HIC BF.
tert-Butyl hydroperoxide (t-BHP) was used as a chemical oxidant to promote oxidation of methionine residues of NIST mAb.
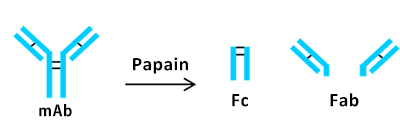
Subsequently, papain was used for the preparation of two Fab fragments and one Fc fragment.
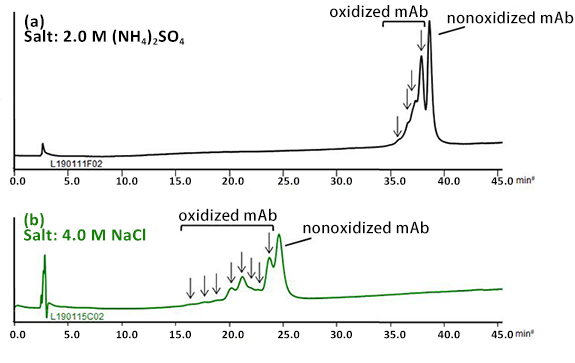
BioPro HIC BF column demonstrated the separation of oxidized mAb from the nonoxidized mAb using a low flow rate and shallow gradient slope.
In the chromatogram (a), earlier eluting four peaks were assumed to be derived from species that would have oxidized methionine residues on the mAb. The oxidation of sulfide side chains on methionine residues might result in conformational changes.
By using sodium chloride instead of ammonium sulfate, better resolution was achieved with a short analysis time (b).
| Column | BioPro HIC BF 4 µm, 100 X 4.6 mmI.D. |
|---|---|
| Eluent | A) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) containing salt B) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) 40-80%B (0-40 min), 80%B (40-45 min) |
| Flow rate | 0.3 mL/min |
| Temperature | 25°C |
| Detection | UV at 280 nm |
| Injection | 5 µL (1.0 mg/mL) |
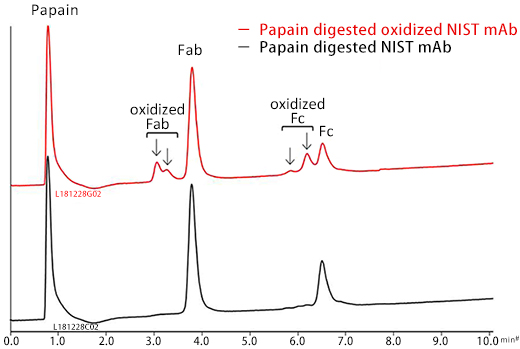
Papain digested NIST mAb samples were analyzed. Fab and Fc peaks were observed on analysis of the nonoxidized mAb sample.
Peaks that would correspond to oxidized Fab and Fc were observed on the analysis of papain digested oxidized mAb sample. The oxidized species eluted earlier than the nonoxidized species. *
| Column | BioPro HIC BF 4 µm, 100 X 4.6 mmI.D. |
|---|---|
| Eluent | A) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) containing 2.0 M (NH4)2SO4 B) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) 40-80%B (0-10 min) |
| Flow rate | 1.0 mL/min |
| Temperature | 25°C |
| Detection | UV at 280 nm |
| Injection | 5 µL (0.5 mg/mL) |
*Journal of Chromatography A, 2008, 1214, 81-89

Hydrophobic interaction chromatography (HIC) is a technique used to separate proteins such as antibodies by hydrophobic interactions between proteins and stationary phase. The mobile phase is typically an aqueous buffer with high concentration. Proteins are adsorbed to the stationary phase at high concentration of salt, and elute in the order of increasing hydrophobicity by decreasing the salt concentration. Unlike reversed-phase, proteins can be separated without any denaturation, thereby maintaining its activity.
On this page, we introduce the factor affecting separation of HIC.
The buffer containing (NH4)2SO4 is often used as mobile phase of HIC because (NH4)2SO4 has strong salting-out effect. The higher the concentration of initial (NH4)2SO4, the stronger retention of proteins, so a buffer with high salt concentration is effective for separation of the low hydrophobic proteins with weak retention.
NaCl and CH3COONH4 are also used as salts. The separation selectivity vary with the type of salt in some cases (see chromatograms above), so changing the type of salt is also effective when the separation is poor. However, these salts are used very high concentration to gain retention comparable to (NH4)2SO4. It is need attention that precipitation of salts in the buffer and damage of LC system.
YMC’s column for hydrophobic interaction chromatography, BioPro HIC BF, is designed to high hydrophobicity of stationary phase. Therefore, it enables to analyze low hydrophobic proteins that can’t be retained using other commercial columns even in lower salt concentration buffer or low salting-out effect salts buffer.
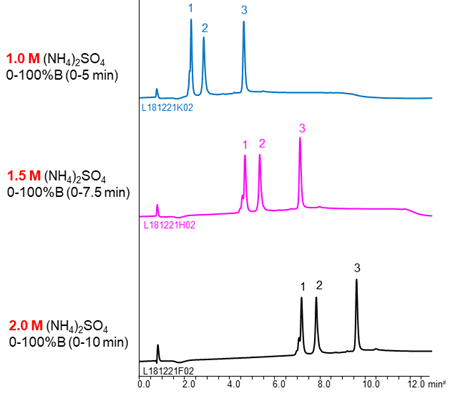
| Column | BioPro HIC BF 4 µm, 100 X 4.6 mmI.D. |
|---|---|
| Eluent | A) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) containing salt B) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) 0.2 M/min (The gradient slope is same.) |
| Flow rate | 1.0 mL/min |
| Temperature | 25°C |
| Detection | UV at 280 nm |
| Injection | 5 µL (0.5 mg/mL) |
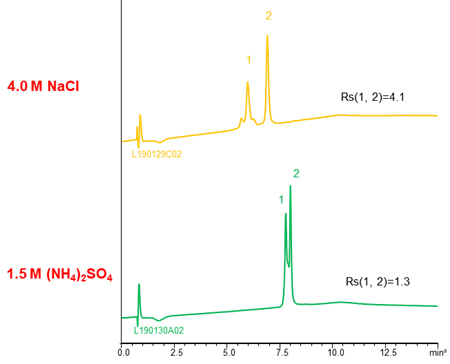
| Column | BioPro HIC BF 4 µm, 100 X 4.6 mmI.D. |
|---|---|
| Eluent | A) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) containing salt B) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) 0-100%B (0-10 min), 100%B (10-15 min) |
| Flow rate | 1.0 mL/min |
| Temperature | 25°C |
| Detection | UV at 280 nm |
| Injection | 10 µL (0.25 mg/mL) |
Using more shallow gradient make improve separation.
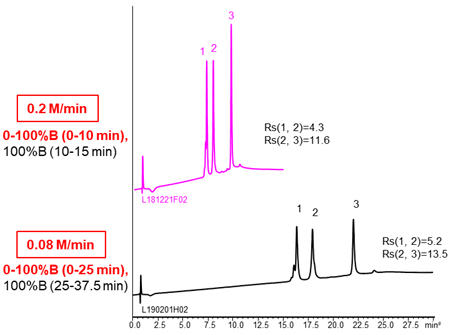
| Column | BioPro HIC BF 4 µm, 100 X 4.6 mmI.D. |
|---|---|
| Eluent | A) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) containing 2.0 M (NH4)2SO4 B) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) |
| Flow rate | 1.0 mL/min |
| Temperature | 25°C |
| Detection | UV at 280 nm |
| Injection | 10 µL (0.5 mg/mL) |
In HIC mode, the higher temperature, the longer retention time of proteins. It assume that the hydrophobic area interacted with stationary phase become large by changing the structure of proteins with temperature increased, so the hydrophobic interaction become strong.
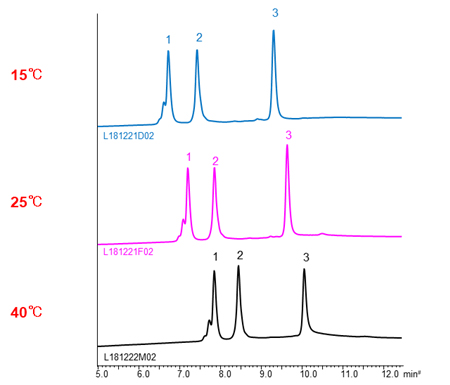
| Column | BioPro HIC BF 4 µm, 100 X 4.6 mmI.D. |
|---|---|
| Eluent | A) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) containing 2.0 M (NH4)2SO4 B) 100 mM NaH2PO4-Na2HPO4 (pH 7.0) 0-100%B (0-10 min), 100%B (10-15 min) |
| Flow rate | 1.0 mL/min |
| Detection | UV at 280 nm |
| Injection | 5 µL (0.5 mg/mL) |
近期留言